We know that helping to improve the lives of people living with serious medical conditions takes more than scientific innovation alone – it also requires close collaboration with patient and advocacy communities.
Our dedicated Global Patient Affairs team collaborates with groups across BioMarin and a broad range of community organizations in regions around the world. Together we help ensure that the lived experiences and unique needs of those affected by rare genetic conditions are integrated into our business, during research and development of potential new treatments as well as after a medicine is approved.
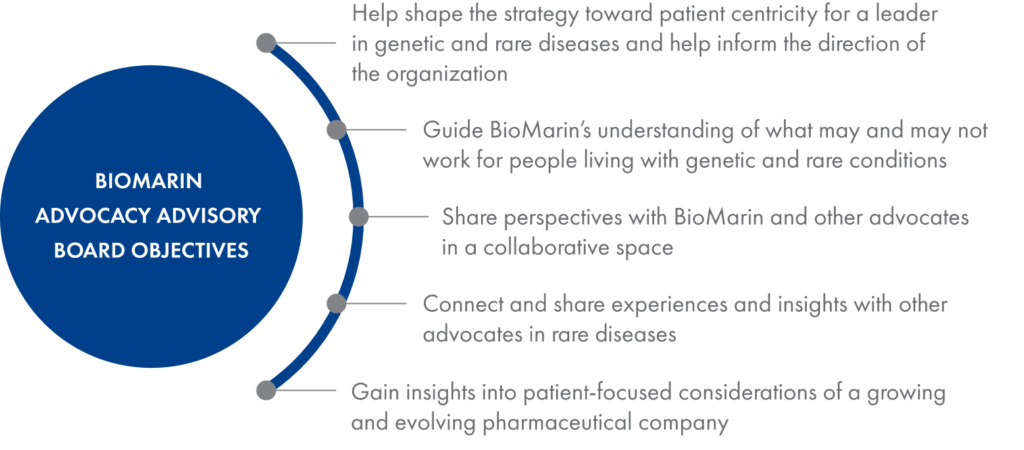
To help ensure that patients are always at the heart of our business decisions, we assembled the BioMarin Advocacy Advisory Board, which consists of eight patient advocates from Europe, the United States and Canada. This group provides insights and counsel around how we collaborate with patients, key activities, and strategies that may extend beyond a particular disease area or therapy.
The board includes advocates for communities living with genetic conditions of direct interest to BioMarin as well as people representing other conditions currently less connected to BioMarin activities.
Co-created with a multi-national steering committee of patient advocacy leaders, our annual Europe and Canada Patient Advocates Summit (ECPAS) aims to:
In 2023, ECPAS brought together 40 participants from 20 countries and focused on some of the biggest challenges facing the rare disease patient community, including:
These topics will be the focus of more in-depth discussion at ECPAS 2024, where the aim is to identify effective advocacy practices that can help realize important changes for people affected by life-impacting rare conditions.
Patients, advocates and caregivers are uniquely positioned to characterize the current state of care, unmet needs, treatment priorities and preferences for their condition.
We take a deliberate, systematic approach to help ensure that patients’ perspectives are captured and meaningfully incorporated into the development and evaluation of treatments – from the time of the earliest ideas for a medicine and for as long as that medicine may be available to patients. Listening to patients as experts in the lived experience of their conditions is essential to our success and helps us have more meaningful, well-informed interactions with health authorities, payers and other stakeholders.