Clinical Data
Efficacy
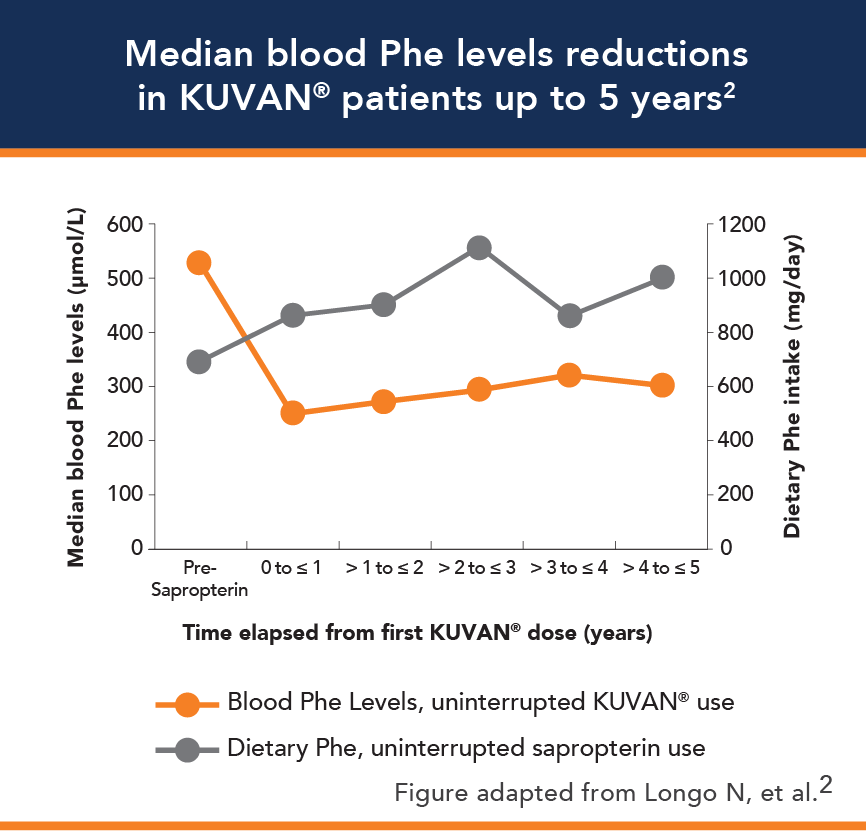
Results of clinical trials demonstrated the efficacy of KUVAN® to reduce blood Phe levels and to increase dietary Phe tolerance.1
PKU-001 was a multicentre, open-label, uncontrolled clinical trial of 489 PKU patients aged 8-48 years who had baseline blood Phe levels ≥450μmol/L and were to receive KUVAN® 10mg/kg/day for 8 days; while PKU-003 was a multicentre, double-blind, placebo-controlled trial of PKU patients who responded to KUVAN® in PKU-001. After a washout period from PKU-001, patients were randomised equally for 6-week treatment with KUVAN® 10mg/kg/day or placebo.1
The results showed that:1
- KUVAN® 10mg/kg/day significantly reduced blood Phe levels as compared to placebo.
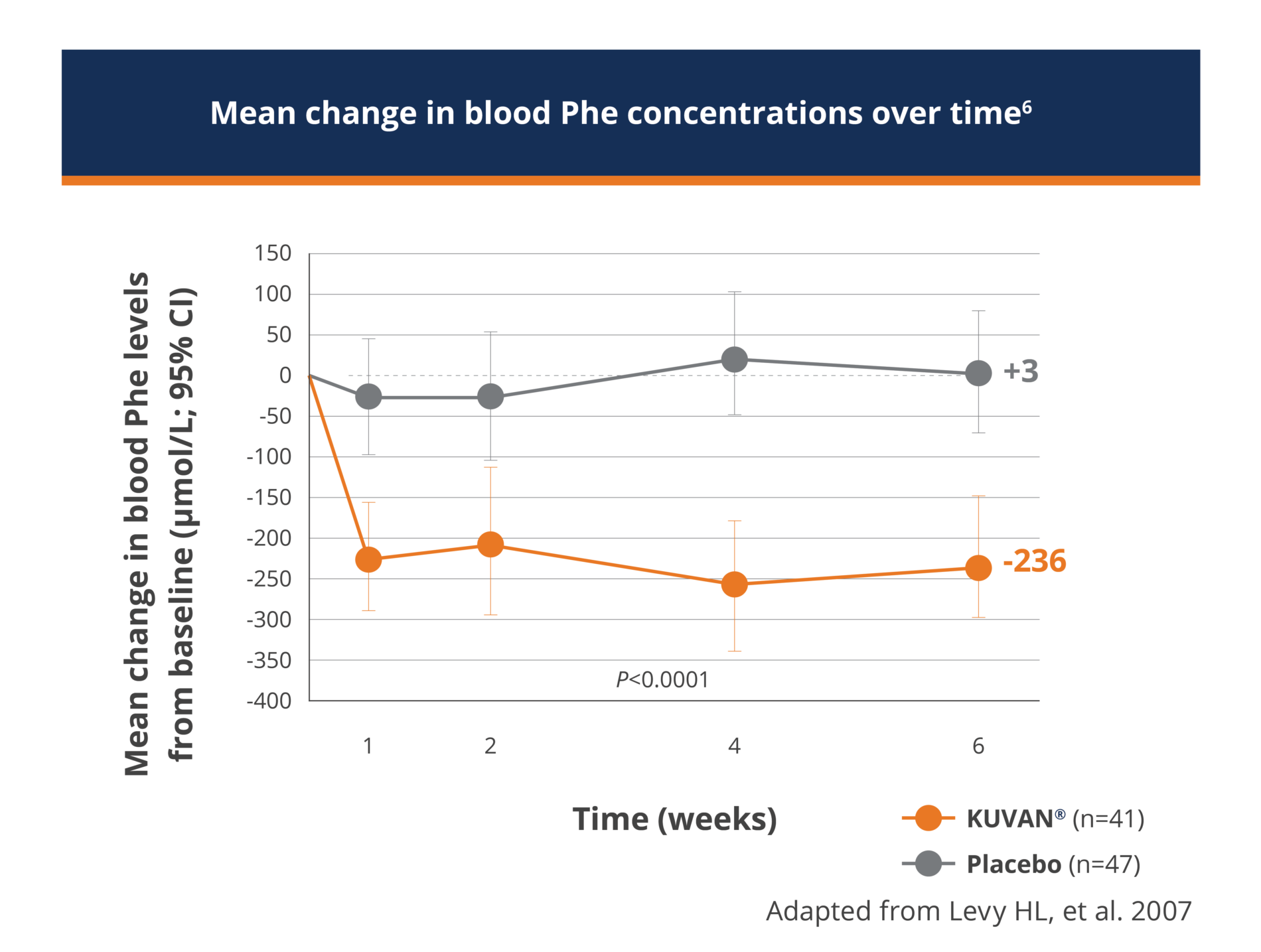
- The baseline blood Phe levels for the KUVAN®-treated group and the placebo group were similar, with mean (±SD) baseline blood Phe levels of 843 (±300) μmol/L and 888 (±323) μmol/L, respectively.
- The mean (±SD) decrease from baseline in blood Phe levels at the end of the 6 week study period was 236 (±257) μmol/L for the KUVAN® treated group as compared to an increase of 3 (±240) μmol/L for the placebo group (p<0.001)
For patients with baseline blood Phe levels ≥600μmol/L, 42% (13/31) of those treated with KUVAN® and 13% (5/38) of those treated with placebo had blood Phe levels <600μmol/L at the end of the 6-week study period (p=0.012).