
KUVAN® PBS Prescribing Codes
A quick guide to look up the KUVAN® PBS authority prescribing codes – now available for adults with PKU from 1 April 2023
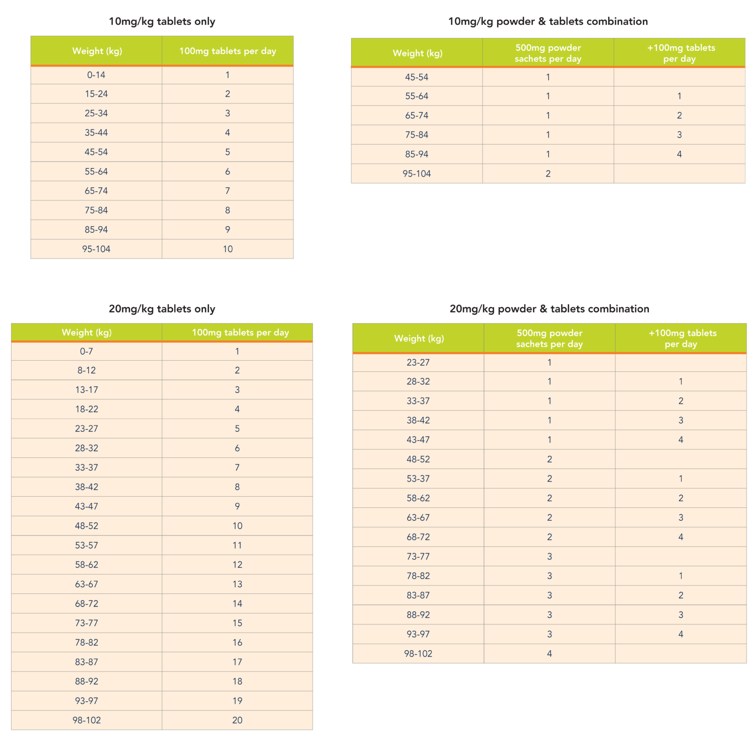
KUVAN® Dosing Information
A quick guide to the recommended number of KUVAN® 100mg tablets and/or 500mg powder to prescribe, depending on your patient’s body weight

KUVAN® product information
The Product Information (PI) provides health professionals with a summary of the scientific information relevant to the safe and effective use of this prescription medicine. This information is intended for use in Australia only.




