VOXZOGO® PBS Prescribing Codes
A Quick guide to the VOXZOGO® PBS authority prescribing codes
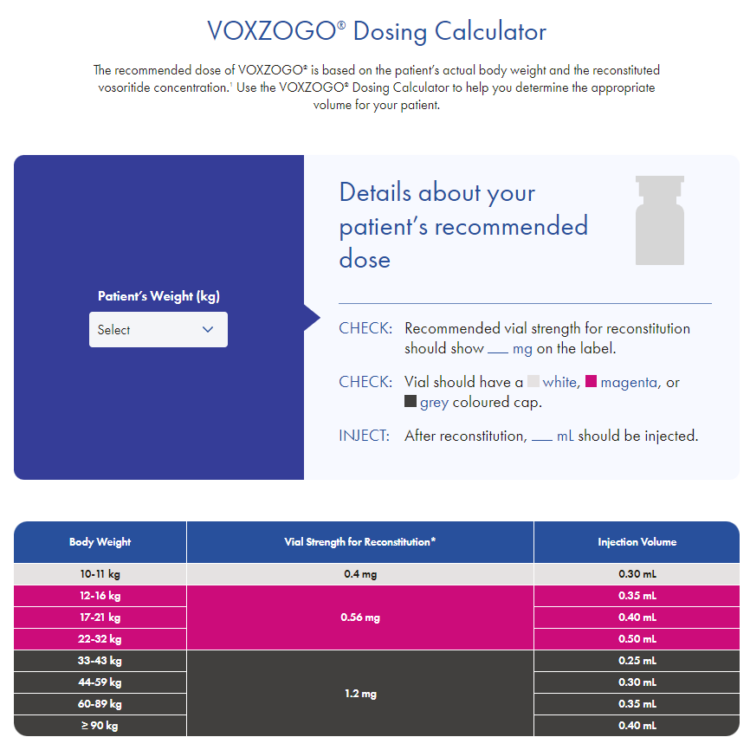
VOXZOGO® Dosing Calculator
Select the correct dose for your patient and learn how it is administered

VOXZOGO® Dosing and Administration Guide
This guide will familiarise you with administration so you can help patients and caregivers feel comfortable with daily injections.