Adverse reactions that occurred in ≥5% of patients treated with VOXZOGO and at a rate greater than placebo in Study 11,*
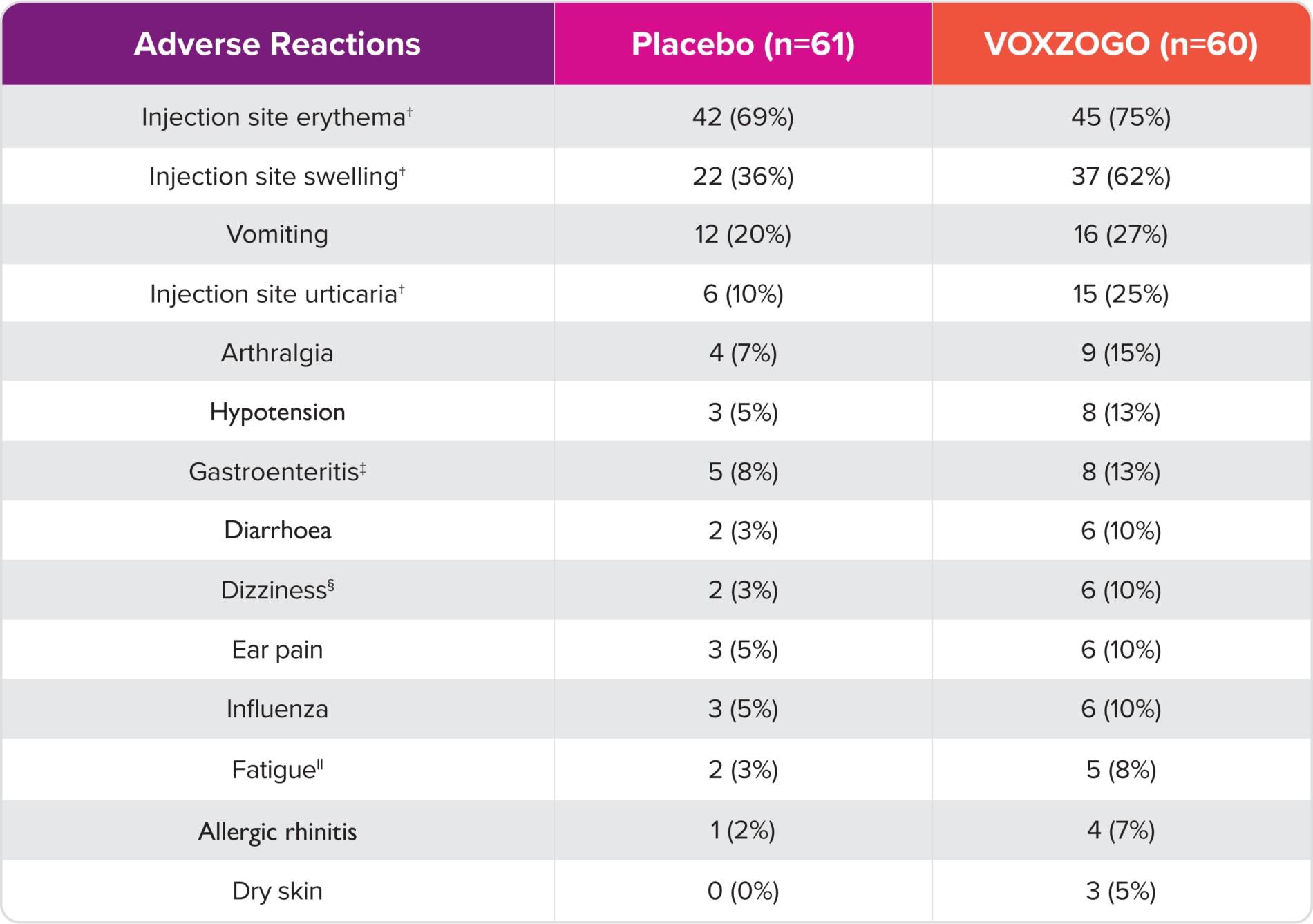
*Includes adverse reactions occurring more frequently in the VOXZOGO arm and with a risk difference of ≥5% (ie, difference of >2 subjects) between treatment arms.
†Injection site reactions occurring more frequently in VOXZOGO-treated subjects than placebo.
‡Includes the preferred terms: gastroenteritis and gastroenteritis, viral.
§Includes the preferred terms: dizziness, presyncope, procedural dizziness, and vertigo.
||Includes the preferred terms: fatigue, lethargy, and malaise.